Our Services covered the preclinical work with facilitating the drug discovery research work with dedicated multi disciplinary team in area of toxicology, pharmacology and bio-analysis on step by step for discovery of products or new compounds.
We are engaged in providing highly reliable and cost-effective Laboratory Testing Services. In addition to this, we undertake bio-analysis, Toxicological & Pharmacological Studies at our advanced facility.
Being a government-approved testing services, we are known for responsibly carrying out testing of various food materials, drug and toxicological study. The ITC Labs is NABL & FDA accredited organization.
We are offering following services as per standard regulatory guidelines
- Acute Toxicity Studies, (Oral, dermal, subcutaneous, intramuscular, intra peritoneal)
- Sub-Acute & Chronic Toxicity Studies,(Oral, dermal, subcutaneous, intramuscular, intra peritoneal)
- Irritation Study (Dermal route)
- Reproductive & Developmental Toxicity
- Carcinogenicity & Mutagenicity.
- Furthermore, we render Supplementary Toxicity Studies, Invitro Toxicity Study
- Guinea Pigs sensitization Studies (M&K; and Buehler’s Methods)
- Acute Inhalation Toxicity Studies (rats)
Toxicological studies
Acute toxicology means that the adverse effects of a substance/ any new compounds that result either from a single exposure or from multiple exposures in a short period of time (usually less than 24 hours). To be described as acute toxicity, the adverse effects should occur within 14 days of the administration of the substance.
Subacute toxicity
Adverse effects occurring as a result of repeated daily dosing of a chemical, or exposure to the chemical, for part of an organism’s lifespan (usually not exceeding 10%). With experimental animals, the period of exposure may range from a few days to 28 days.
Chronic toxicity
Chronic toxicity is the development of adverse effects as the result of long term exposure to a toxicant or other stressor. It can manifest as direct lethality but more commonly refers to sublethal endpoints such as decreased growth, reduced reproduction, or behavioral changes such as impacted swimming performance.
ITC Labs lab is offering the toxicological studies as per regulatory requirements GLP, OECD, EPA and Schedule Y guidelines.
We are testing the compounds/ New chemical entity / products for toxicological study through various routes of administration as per annexure-I
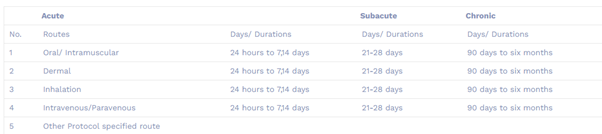
- We followed the various guidelines for acute toxicity, Subacute and chronic toxicity
- 1.1 Acute Oral Toxicity – Acute Toxic Class Method (OECD 423)Acute toxicity for oral, cosmetic dermal products
- 1.2 Acute Oral Toxicity- Up-and-Down-Procedure (UDP; OECD425)
- 1.3 Acute oral toxicity for LD50 determination
- Maximum Tolarence Dose Study for oral, cosmetic dermal products
- Acute Tolarence Dose (ATD) Study for oral, cosmetic dermal products
- No observed Adverse Effect Level Study (NOAEL) for oral, cosmetic dermal products
- Acute Inhalation study (OECD 403)
ITC Labs lab working in area of toxicological studies related to skin and cosmetic products through OECD, EPA and schedule Y guidelines
Skin irritation study (OECD404) : This method provides information on health hazard likely to arise from exposure to liquid or solid test substance by dermal application. This Test Guideline recommends sequential testing strategies, which include the performance of validated and accepted in vitro or ex vivo tests for corrosion/irritation.
Eye Irritation/Corrosion Study (OECD 405)
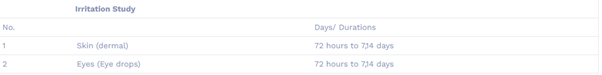
Eyes irritation in Rabbits
Skin sensitization study in Guinea pigs (OECD 406)

Skin sensitization study by Local Lymph Node Assay (OECD 429)
The LLNA provides an alternative method for identifying skin sensitising chemicals and for confirming that chemicals lack a significant potential to cause skin sensitisation
Genotoxicity studies
- Bacterial Reverse Mutation Assay (AMES Test)
- Mammalian Erythrocyte (Bone Marrow) Micronucleus Test (In-vivo)
- In-vitro Mammalian Cell Micronucleus Test
- Mammalian Bone Marrow Chromosome Aberration Test (In-vivo)
- In-vitroMammalian Cell Chromosome Aberration Test
- In-vitro Mammalian Cell Gene Mutation Assay (Mouse Lymphoma Assay)
Reproduction and Developmental Toxicity Studies
- Reproduction Toxicity Studies (Male fertility and Female Segment. I Studies) in rats
- Teratology Studies (Seg. II Embryo-foetal development) in rats and rabbits
- Peri- and post-Natal developmental Studies (Segment. III) in rats
Pharmacological Study
Pharmacological testing square measure used once one needs to judge if a substance or plant extract is biologically active. Pharmacological Testing ways for medicine performing on the Peripheral system. Our Pharmacological testing services enable us to predict biological effects of latest molecular entities. Safety Pharmacological testing studies square measure studies that investigate potential undesirable pharmacodynamic effects of a substance on physiological functions in relation to exposure at intervals the therapeutic vary or higher than. These Pharmacological testing studies studies ought to be designed to spot undesirable pharmacodynamic properties of a substance which will have connexion to its human safety, to judge adverse pharmacodynamic and pathophysiological effects discovered in materia medica and clinical studies.
We also carry out study and research on drugs & pharmacological testing, which is called the Pharmacological Study Services . Everything about the drugs is carefully studied by us and we also conduct various experiments to substantiate our study. We also carry out research activities to find out how the living tissues and the organisms function. Their scope for modification by the chemical substances is also studied. The pharmacological study is divided into two streams pharmacodynamics and the pharmacokinetics.
Interstellar testing centre Laboratory has a wide range of services dedicated to early phase drug selection and development process , where test item quantities, time to results and price are optimized for fast and robust decision making.
The laboratory have established various in vitro / in vivo models which allow evaluating the efficacy of your molecule, new compound/ products and establishing PK-PD relationship. Our scientific approach derived from diversely trained group of scientists at pharmaceutical and biotech industries, helps our clients fast track efficacy and safety testing to support lead identification & optimization. The routinely employed models can be fully integrated with histology, pathology, immunohistochemistry, flow cytometry , and behavioral study
We are providing various pharmacological studies
Pain / Inflammation models
- FCA / Capsaicin induced hyperalgesia in rats
- Carrageenan induced paw edema in rats & mice
- Acetic acid / Formalin induced writhing in mice
- TPA / Oxazolone induced ear edema in mice
- TNBS-induced colitis/DSS induced colitis in rats & mice
- Thermal & mechanical pain modules ( Tail Flick model, Hot plate, Plantar test in rats & mice )Neuropathic pain models
Metabolic & Chronic Disorders
- Diabetic Models
- Obesity Models
- Digestive orders, etc.
- Neurological model (TBI, Memory and sensory loss)
- Wound model ( Excision & Incision, Burn injury, Type of Burn injury)
Other pharmacology studies
- Screen of Herbal products for medicinal uses
- Dermal permeability study of drug in rats
- Immunomodulatory activity of compound
- Hypersensitivity and anti hypersensitivity activity study
- In vitro haemolysis test
- Oxidative stress study.
Absorption, Disposition, Metabolism, Elimination and Toxicity (ADMET) studies
Selection of drug candidates with the best ADMET properties should enhance the probability of clinical success. The laboratory offers the complete package of ADMET studies which are critical for clinical success. In vitro human-based experimental system used in combination with in vivo animal systems, using animal species relevant to humans, represent the best approach to assess these important drug-like properties before clinical trials.
Our aim is to design the studies that are necessary for your molecule in the most efficient and cost effective way. All in vitro and in vivo studies are performed under strict quality control.
In vitro ADME studies
In vitro ADME screens provide critical information for lead optimization and selection of the appropriate species for conduct of toxicological studies. Elucidation of the interaction of a test substance under investigation using human cytochrome(s) P450 (CYP) and other drug-metabolizing enzymes is important for predicting its potential for interaction with other drugs which is of great relevance in the planning of clinical studies. In vitro ADME studies offered by laboratory are follow
Absorption studies
- Parallel Artificial Membrane Permeability Assay (PAMPA)
- Cell permeability assays (CaCO2 and MDCK cells)
- Everted Gut Sac Permeability Assay
Distribution
- Plasma Protein Binding –mouse, rat, rabbit, dog and human using equilibrium dialysis method
- Plasma / Whole blood stability – mouse, rat, rabbit, dog and human
- Blood to plasma ratio
Metabolism
- Drug – Drug Interaction studies
- Cytochrome P450 Inhibition (Probe Based)
- Cytochrome P450 Induction in animal species
Metabolism
In-Vivo ADME Studies
Pharmacokinetics
Routine Pharmacokinetic Studies using various routes of administration:



